Background:
Autologous hematopoietic cell transplantation (HCT) is a standard of care after induction therapy for multiple myeloma (MM). A key step in this procedure is the mobilization and collection of hematopoietic progenitor cells (HPC). HPC mobilization typically uses G-CSF with or without the CXCR4 antagonist Plerixafor (P). Although P increases the effectiveness of mobilization and may reduce the number of apheresis sessions, it is associated with significantly increased cost per dose. There is significant heterogeneity to HPC collection algorithms across institutions and there is no clear standard. At the Cleveland Clinic, our HPC collection algorithm was updated in an effort to reduce the use and associated cost of P. The criteria for initiating P became more stringent and far fewer patients were initiated on P prior to their first session of HPC collection apheresis. Prior to October 2017, patients were initiated on P if any 1 of the following criteria were met: age>60 years, prior treatment with melphalan or other alkylating therapy, prior treatment with lenalidomide >2 cycles, extensive bone marrow disease, or platelet count <100K. Starting October 1, 2017, patients were preemptively initiated on P prior to first collection only if all 3 of the following criteria were met: age > 65 years, lenalidomide > 6 cycles, and platelet count < 100K. However, P was added after the first apheresis session if peripheral blood CD34+ cell count was <20/uL or HPC yield was unsatisfactory (<2 x 10^6 CD34+ cells). Furthermore, target CD34 yield was decreased from 5 to 4 x 10^6 CD34+ cells. Herein, we perform a comparative resource utilization analysis of HPC collection at our center.
Methods:
We retrospectively collected HPC mobilization data on patients undergoing HCT for MM from 1/12014 to 12/31/2020. We determined the number of P doses, number of apheresis sessions, and HPC collection totals per patient. Patient characteristics were summarized in median, interquartile range (IQR), or frequencies and percentages as appropriate. Fisher's exact test and Wilcoxon rank sum test were used to compare variables in patients undergoing mobilization prior to versus after 10/1/2017. All tests were two-sided and p-values of 0.05 or less were considered statistically significant.
Results:
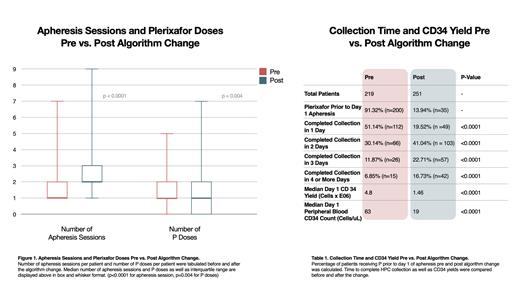
219 patients underwent HPC mobilization from 1/1/2014 to 9/30/2017 and 251 patients underwent mobilization after algorithm change from 10/1/2017 to 12/31/2020. As anticipated, the protocol change decreased the number of patients receiving P prior to their first apheresis session. 91% received P prior to the first apheresis session before the change while 14% received this initial dose of P after the change. Prior to the change 51% of patients completed collection in 1 day, 30% completed in 2 days, 12 % completed in 3 days, and 7% completed in 4 days or more. These figures changed to 20%, 41%, 23%, and 17% respectively following the change (p<0.0001) Median day 1 peripheral blood CD34 count decreased from 63 to 19 cells/uL while median day 1 CD34 collection yield fell from 4.8 to 1.46 x 10^6 after the change (p<0.0001) (Table 1). Total CD34 collection yield also decreased from a median of 6.64 to 4.6 x 10^6 CD34+ cells (p<0.0001). However, the target yield was decreased from 5 to 4 x 10^6 CD34+ cells as described above. The number of doses of P per patient decreased after the change (pre-change median dose / patient = 1, IQR = 1-2) (post-change median dose / patient = 1, IQR = 0-2) (p=0.004). The average number of apheresis sessions required per patient increased following the change (pre-change median apheresis sessions = 1, IQR = 1-2) (median post change apheresis sessions = 2, IQR = 2-3) (p<0.0001) (Figure 1).
Conclusion:
Changes to our institutional HPC mobilization algorithm led to a substantial decrease in the use of preemptive P prior to day 1 of collection by design. The resulting decrease in total doses of P per patient was smaller than expected, although still statistically significant. However, this was offset by a concomitant significant increase in the number of apheresis sessions required per patient despite a lower collection target. Based on these results, a cost analysis is ongoing in order to re-evaluate our institutional algorithm.
Disclosures
Sauter:Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA.: Research Funding; Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, Ono Pharmaceuticals, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK.: Consultancy. Hamilton:Incyte: Other: ad hoc consultancy; NKARTA: Other: ad hoc advisory board; Kadmon/Sanofi: Other: advisory board; Equilium: Other: ad hoc advisory board; Therakos: Honoraria; Angiocrine: Other: DSMB; Rigel: Other: Ad hoc advisory board; CSL Behring: Other: Adjudication committee. Khouri:Janssen: Consultancy, Honoraria; GPCR Therapeutics: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal